Exploring the Chemical Compositions of Meteors

Introduction to Meteor Chemistry
Meteors, often referred to as shooting stars, are fascinating celestial phenomena that occur when meteoroids enter the Earth's atmosphere. As these objects burn up upon entry, they create a stunning display of light and color. However, the science behind meteors goes far beyond their visual appeal. Understanding their chemical compositions is crucial for insights into the formation of our solar system and the nature of other celestial bodies.
Key Chemical Elements
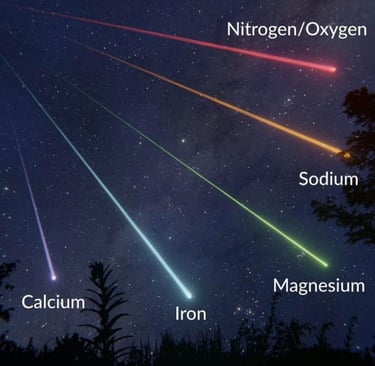
The primary chemical compositions of meteors consist of several elements, including calcium, iron, magnesium, sodium, nitrogen, and oxygen. Each of these elements plays a significant role in the makeup of meteors. For instance, calcium and magnesium are abundant in various types of meteoroids, especially those that originate from asteroids. Iron, on the other hand, is frequently found in metallic meteors, which can withstand greater temperatures during their atmospheric descent.
The Role of Nitrogen and Oxygen
Among the remaining elements, nitrogen and oxygen contribute interesting attributes to meteor chemistry. While nitrogen is an inert gas found in the atmosphere, its presence in meteors can offer clues about the environment from which these celestial objects originated. Similarly, oxygen is a vital component of many minerals and compounds that can be found in meteoric material. Studying these gaseous elements can help scientists decipher the transformation of these materials as they travel through the universe.
In conclusion, the chemical compositions of meteors—comprising elements like calcium, iron, magnesium, sodium, nitrogen, and oxygen—provide valuable information about their origins and the broader cosmic processes at play. As researchers continue to explore the fascinating realm of meteoric science, the knowledge gained may illuminate the mysteries of our solar system and beyond.
